Researchers begin clinical trial to assess schistosomiasis vaccine
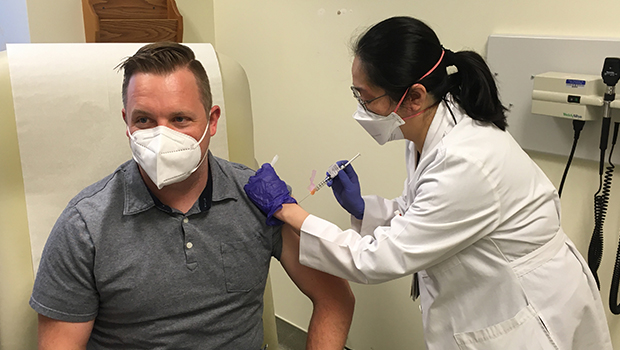
Alex Stevenson receives the first-ever shot of the SchistoShield investigational vaccine at KPWHRI on May 23, 2022. (Photo credit: Amelia Apfel)
Participant in KPWHRI trial receives world’s first-ever injection of investigational SchistoShield vaccine
An estimated 200 million people are infected with schistosomiasis and an additional estimated 800 million people are at risk of acquiring the disease. Schistosomiasis, caused by parasitic worms, is estimated to cause 280,000 deaths annually in 78 countries. Infectious Diseases Clinical Research Consortium (IDCRC) researchers are conducting a clinical trial to assess the safety and effectiveness of an investigational schistosomiasis vaccine. The trial could help pave the way for the world’s first vaccine against the disease, offering a safe and cost-effective option to lower its prevalence worldwide and prevent deaths.
Currently, schistosomiasis is mainly treated with the drug praziquantel. However, the drug has been found to be insufficient due to its limited impact on the reduction of disease transmission and infection rates remain high.
“Schistosomiasis remains a major public health problem due to its high levels of morbidity and mortality in many parts of the world,” said Lisa Jackson, MD, MPH, senior investigator at Kaiser Permanente Washington Health Research Institute and study principal investigator. “An effective schistosome vaccine could offer sustainable reduction in both the prevalence and transmission of the disease, saving lives around the world.”
This new trial will assess the safety of the Sm-p80 + GLA-SE (SchistoShield®) study vaccine. The research will measure indicators of immunity to schistosomiasis. The study will also assess whether the vaccine prompts an immune response, leading to the generation of antibodies that can prevent infection.
Participants will include healthy people ages 18 through 55 years old. Volunteers will be assigned to 1 of 5 study groups, based on when they enroll in the study. Each group will include 9 participants. Group A will receive the study vaccine without the adjuvant (a substance that increases the body’s response to vaccines), at a dose of 100 micrograms (μg) of Sm-p80, to test whether the adjuvant acts to increase immune responses. Groups B, C, D, and E will receive the study vaccine with the adjuvant. The dose of the Sm-p80 protein will be 10 μg in Group B, 30 μg in Groups C and D, and 100 μg in Group E to find out what dose leads to the best immune responses and side effect profile. The study will run over a 15-month period.
Participating in the trial
Healthy people age 18 through 55 years old may be eligible to enroll in this study, which will run over a 15-month period and require 12 or 13 study visits at the KPWHRI research clinic in downtown Seattle. To learn more about this study in Seattle, email KPWA.vaccine@kp.org with your full name and date of birth and the best phone number and time to reach you. (NOTE: Enrollment is now closed.)
Visit ClinicalTrials.gov for additional details. The study, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health, is being conducted through the IDCRC.
The study site is Kaiser Permanente Washington Health Research Institute — Vaccines and Infectious Diseases (UM1AI148576).
About Infectious Disease Clinical Research Consortium (IDCRC)
This activity is supported by the Infectious Diseases Clinical Research Consortium (IDCRC) through the National Institute of Allergy and Infectious Diseases (NIAID) (NCT05292391). The IDCRC, consisting of the Vaccine Treatment and Evaluation Units (VTEUs) and the IDCRC Leadership Group, was formed in 2019 to support the planning and implementation of infectious diseases clinical research that efficiently addresses the scientific priorities of NIAID. The consortium includes infectious diseases leaders and clinical researchers from Emory University, University of Maryland School of Medicine, Baylor College of Medicine, Cincinnati Children’s Medical Center and University of Cincinnati, FHI360, Fred Hutchinson Cancer Research Center, Johns Hopkins University, Kaiser Permanente Washington Health Research Institute, New York University, Saint Louis University, Vanderbilt University Medical Center, University of Alabama at Birmingham, University of Rochester, University of Washington, and NIAID. For more information about the IDCRC, please visit IDCRC.org.
1st vaccine shot
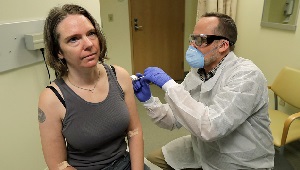
COVID-19 vaccine trial volunteer on the shot heard 'round the world
On March 16, 2020, Jennifer Haller became the first person to be injected with a COVID-19 vaccine in a clinical trial.
Research

‘Fighting back’ against COVID-19: Remembering a historic trial
Lisa Jackson, MD, MPH, Kaiser Permanente Washington senior investigator, recounts the genesis of a groundbreaking vaccine.
New clinical trial

NIAID-sponsored study evaluates second COVID-19 booster shots
KPWHRI joins trial that includes multiple variant vaccines.



