Coronavirus vaccine trial now includes older people

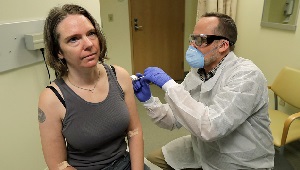
Dr. Lisa Jackson, right, a KPWHRI senior investigator, confers with pharmacist Michael Witte, left, on March 16 in Seattle before the first injections of the experimental COVID-19 vaccine were given. (AP Photo/Ted S. Warren)
Led by Dr. Lisa A. Jackson, first-ever trial of any experimental vaccine candidate for COVID-19 virus has expanded
Older adults are now being enrolled in the first trial of the first experimental vaccine candidate for the COVID-19 virus. As the trial expands to include a total of 105 healthy adults, Kaiser Permanente Washington Health Research Institute (KPWHRI)’s vaccine research team will enroll 20 healthy Seattle-area participants age 71 years and older.
Another 10 participants in that age group will be enrolled at NIAID’s Vaccine Research Center clinic at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD, according to a news statement from the National Institute of Allergy and Infectious Diseases (NIAID), which funds the trial. And 30 participants age 56–70 years will enroll at the NIH and Emory University in Atlanta. But no one in the 56–70-year age group will enroll at KPWHRI.
Disease risk is higher in older people
“Older adults are at high risk of serious infection,” says trial leader Lisa A. Jackson, MD, MPH, senior investigator at KPWHRI. “Expanding the age range in this trial to include them gives us the ability to get a first look quickly at the safety of the vaccine and the immune responses to it in this group.” And expanding the trial to Atlanta and Bethesda, not only Seattle, ensures that it can move forward without dependence on a single geographic location.
On March 16, KPWHRI gave the first-ever injection of an investigational vaccine against the 2019 novel coronavirus, also known as SARS-CoV-2, which causes COVID-19. Since launching the trial of the mRNA-1273 vaccine, KPWHRI has finished enrolling 28 participants age 18–55 here. Meanwhile, 17 are being enrolled at Emory University, for a total of 45 in that age group.
Safety first
Study investigators, including Dr. Jackson, have conducted regular safety reviews and have not identified any significant safety issues among any participants in this younger age group. Regular safety reviews will continue as older adults are enrolled.
The vaccine was produced by Moderna, Inc. using a new process that is much faster than older methods of making vaccines. The vaccine includes a short segment of messenger RNA that is made in a lab and codes for the viral spike protein. It does not include any form of live virus, and the trial will not expose participants to the virus.
The trial is a small “phase I” test in a 3-phase process examining the potential vaccine. In this phase, Dr. Jackson and other researchers at KPWHRI, Emory, and NIH are testing safety and antibody production. This phase I trial is not studying the effectiveness of the vaccine in preventing coronavirus infection. That will come at a later phase of the research. Development of a vaccine typically takes up to 18 months.
KPWHRI’s vaccine research team began preparations for the possibility of a trial as soon as they got the call on January 28. The team has expertise in conducting these kinds of trials, including testing other investigational vaccines against “swine” and “bird” flu. KPWHRI is part of the NIAID’s Infectious Diseases Clinical Research Consortium, which helps develop new and improved vaccines and therapies against infectious diseases. KPWHRI is the only one of the nation’s 9 consortium centers not housed at a university medical center.
The Gates Foundation is funding construction of factories for the 7 most promising vaccine candidates (including the Moderna vaccine in clinical trial at Kaiser Permanente, Emory, and NIH), even though they know only one will end up getting picked to move into production.
If interested in participating?
Healthy adults age 71 or older, who live or work in the Seattle area and think they might be interested in participating in this trial, can email kpwa.vaccine@kp.org for more information. The response has already been strong, and the team may not be able to respond quickly.
To be eligible, trial participants can’t have certain health conditions that affect the immune system, and they can’t be taking medications that affect the immune system. Having met their goals for younger participants, the research teams are no longer enrolling people age 55 and younger.
By Rebecca Hughes
research
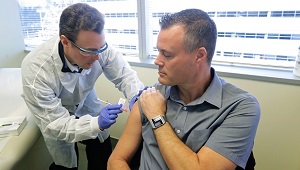
Kaiser Permanente launches first coronavirus vaccine trial
On March 16, 4 volunteers received an injection of an mRNA vaccine for COVID-19 in an NIH-funded trial in Seattle.
research

Clinical trial of H7N9 bird flu vaccine starts at KPWHRI
Dr. Lisa A. Jackson leads national trial to explore improving immune responses to the vaccine.
Read it in News and Events.
director's note
Research versus COVID-19
From clinical trials to technical support, Dr. Rita Mangione-Smith describes how Kaiser Permanente Washington researchers are fighting the novel coronavirus.



