Seattle team to build hi-res brain map of Alzheimer’s disease

Dr. Dirk Keene, associate professor of pathology at the University of Washington, with brain specimens from ACT Study participants.
$40.5M NIH-funded project aims to pinpoint cells at root of brain disease—and new therapeutic targets
If scientists understood how Alzheimer’s disease starts, they might have a better handle on how to stop it. That’s the premise behind a new $40.5 million collaborative research center in Seattle to build high-resolution maps of Alzheimer’s patients’ brains and identify how their neurons and other brain cells differ from those of healthy people.
By comparing brain cells across patients with different stages of the disease, the researchers will seek to pinpoint how and where the progressive disorder starts—and, ultimately, to find new targets for therapy. The experiments will rely on postmortem brain tissue from people who took part in these studies and consented to donate their brains to science after their death:
- the UW School of Medicine BioRepository and Integrated Research (BRaIN) laboratory, which supports brain donation from research participants in the Adult Changes in Thought (ACT) study, a collaboration between Kaiser Permanente Washington Health Research Institute (KPWHRI) and the University of Washington (UW) to learn about healthy aging and dementia including Alzheimer's disease; and
- UW Medicine’s NIA-funded Alzheimer’s Disease Research Center.
The National Institute on Aging (NIA) of the National Institutes of Health (NIH) is funding the center over 5 years. The center is headquartered at the Allen Institute, with additional projects based at UW Medicine and KPWHRI. The center’s work will build off methods developed at the Allen Institute and elsewhere through the NIH-funded BRAIN Initiative that use genes switched on in individual brain cells to classify the cells into categories, or cell types.
“The long-running ACT study, with its cohort extending back to 1994, has been at the forefront of today’s realization of the complexity of Alzheimer’s disease in older people,” said Eric B. Larson, MD, MPH, a senior investigator at KPWHRI. “We are excited to partner with the Allen Institute to do a really deep dive into the complex biological underpinnings of Alzheimer’s disease. The work we can do with this award is a scientists’ dream come true for me as founding principal investigator of the ACT study. On behalf of our dedicated participants and Kaiser Permanente Washington, we are excited for this opportunity.”
Plaque-targeting treatments have disappointed
Using these methods to study brains from people across the spectrum of early- to late-stage Alzheimer’s disease should reveal the specific kinds of neurons and other brain cells that are most vulnerable at the beginning of the disease, said Ed Lein, PhD, a senior investigator at the Allen Institute for Brain Science, a division of the Allen Institute, and lead investigator of the new center. Such a detailed understanding of Alzheimer’s origins is desperately needed, as many once-promising treatments—primarily aimed at disrupting the disease’s hallmark plaques of beta-amyloid protein in the brain—have failed to benefit patients. Currently, no therapies exist that can halt the progression of Alzheimer’s, which affects approximately 5.8 million Americans and, together with other dementias, costs the United States an estimated $290 billion every year.
“We’re trying to cure a disease of a complex system we fundamentally don’t understand,” Dr. Lein said. “Historically, the field has focused on the amyloid hypothesis, but that hasn’t panned out for treatment. What’s really needed is to take a fresh look at the basic progression of the disease across the brain, and we now have high-resolution cellular and molecular technologies in place to do just that.”
The data from this center will be openly available to the scientific community at large. The researchers aim both to make direct scientific headway into the root causes of Alzheimer’s disease and to provide a foundational data resource to catalyze progress in treating other neurodegenerative diseases.
The center’s work could also uncover new insights into both the people who have a natural resistance to developing the hallmark amyloid plaques and those who develop these plaques but never develop dementia. Understanding where this natural resistance and resilience arises in the brain could point to new therapeutic pathways.
“Alzheimer’s disease is incredibly complicated, in part because different cells and parts of the brain are differentially affected,” said Bradley Hyman, MD, PhD, a professor of neurology and Alzheimer’s researcher at Harvard Medical School and Massachusetts General Hospital who also serves as an advisor to the new research center. “This complexity makes it very difficult to model in experimental systems, and direct examination of the human brain is without a doubt crucial to understanding the disease. The technology developed at the Allen Institute provides a new approach to unravel these mysteries. I can’t wait to see what they discover.”
Building of foundational brain science
The center’s projects will build off recent and ongoing work led by Dr. Lein and collaborators to generate a cell “census” of the healthy human brain. In that foundational work, the researchers use single-cell technologies originally developed through genomics research to describe brain cell types by the complete set of genes the cells actively use. The new research will apply those techniques to identify how specific brain cell types and their active genes are affected as Alzheimer’s disease progresses—with the ultimate goal of finding new drug targets.
The research teams will analyze cells from multiple brain regions from approximately 100 people, ranging from people with normal cognition and little or no sign of Alzheimer’s disease in the brain to those with late-stage Alzheimer’s dementia.
The new research center will also adapt brain-banking methods to allow the application of modern single-cell technologies to postmortem brains. This will provide samples ready for the analyses in this project and generate a brain tissue repository that can be used for future single-cell studies.
Thanks to research participants
“This work wouldn’t happen without the generous research participants and their dedication to support dementia research,” said C. Dirk Keene, MD, PhD. Dr. Keene is an associate professor of pathology at UW Medicine, an affiliate investigator at KPWHRI, and one of the principal investigators at the center. “We want to honor their incredible gift to science by making sure we can study their brains using the latest, most advanced technology to have the greatest impact for years to come.” The participants include the Kaiser Permanente Washington patients who take part in the ACT study.
This research is supported by the NIA of the NIH under award number U19AG060909.
About the Allen Institute for Brain Science
The Allen Institute for Brain Science is a division of the Allen Institute (alleninstitute.org), an independent, 501(c)(3) nonprofit medical research organization, and is dedicated to accelerating the understanding of how the human brain works in health and disease. Using a big science approach, the Allen Institute generates useful public resources used by researchers and organizations around the globe, drives technological and analytical advances, and discovers fundamental brain properties through integration of experiments, modeling and theory. Launched in 2003 with a seed contribution from founder and philanthropist, the late Paul G. Allen, the Allen Institute is supported by a diversity of government, foundation and private funds to enable its projects. The Allen Institute for Brain Science's data and tools are publicly available online at brain-map.org.
About UW Medicine
UW Medicine is one of the top-rated academic medical systems in the world. With a mission to improve the health of the public, UW Medicine educates the next generation of physicians and scientists, leads one of the world’s largest and most comprehensive biomedical research programs, and provides outstanding care to patients from across the globe. The School of Medicine faculty is second in the nation in federal research grants and contracts with $923.1 million in total revenue (fiscal year 2018) according to the Association of American Medical Colleges. UW Medicine includes Airlift Northwest, Harborview Medical Center, UW Medical Center–Montlake, UW Medical Center–Northwest, UW Neighborhood Clinics, UW Physicians, UW School of Medicine, and Valley Medical Center.
aging and geriatrics
The ACT Study: Looking toward the future
At 25 years, the Adult Changes in Thought Study is committed to science, collaboration, and jokes, writes Erin Bowles
ACT STUDY

The science of aging brains: ACT collaborations grow
A joint KPWHRI-UW program continues to be a rich resource for researchers studying resilience and ways to avoid Alzheimer’s disease and other dementias.
RESEARCH
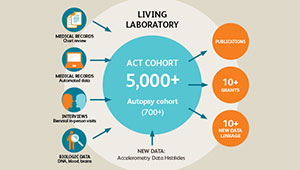
ACT study: Long-running study of aging examines changes in Kaiser Permanente patients over time
Drs. Larson and Crane co-lead Kaiser Permanente-University of Washington collaboration learning how to promote healthy aging.


