Cancer
Research overview
Cancer is complex and can be a devastating diagnosis for individuals and their families. Our researchers work to improve cancer control on many levels, from prevention and screening through treatment and survivorship.
Kaiser Permanente Washington Health Research Institute (KPWHRI) has been studying how to provide life-saving breast cancer screening since 1986. In a novel initiative, researchers used a computer-based registry and collaborations with organizations across the U.S. before electronic health records existed and multi-site studies were common. The program ultimately led to a rich portfolio of studies not only on breast cancer, but also on colorectal, blood, cervical, lung, and thyroid cancers.
“Decades later, Kaiser Permanente Washington is just as committed to developing and evaluating innovations in cancer care,” said Karen Wernli, PhD, KPWHRI senior investigator.
The core of KPWHRI cancer research lies in its participation in many collaborative networks and programs. “Collaboration increases the power of Kaiser Permanente studies and helps us learn from different populations and health care settings,” said Senior Investigator Jessica Chubak, PhD. Examples of these networks include:
Breast Cancer Surveillance Consortium (BCSC)
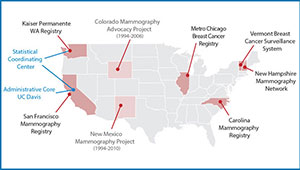
The BCSC identifies strategies that detect aggressive breast cancer early, minimize harms, and reach diverse communities. Results help policymakers and health systems improve breast cancer screening and surveillance outcomes. The Kaiser Permanente Washington Breast Imaging Registry contributes to this national effort and the BCSC Statistical Coordinating Center resides at KPWHRI.
Population-based Research to Optimize the Screening Process (PROSPR)
The PROSPR national consortium conducts research to improve screening for cervical, colorectal, and lung cancers. PROSPR results will help optimize and tailor screening for different subgroups of the population.
Kaiser Permanente Research Bank (KPRB) Cancer Cohort
The KPRB Cancer Cohort is a national resource for understanding genetic, lifestyle, and environmental factors that contribute to cancer etiology and survival. Electronic health record data, stored tissue specimens, detailed treatment data, and the ability to follow patients for recurrence and mortality are accelerating progress toward improved cancer care.
Some of the cancer research group’s current studies include:
- The STEP trial, a pragmatic trial testing different interventions to increase cervical cancer screening, including self-sampling kits available by mail
- The CAFÉ study, which is developing and testing ways to prevent and reduce cancer-related financial hardship
- The LARCH study, which is looking at multilevel interventions to increase adherence to lung cancer screening, including partnership with a patient advisory board
- The VOICE study, which is evaluating outcomes for adolescent and young adult cancer patients after cancer treatment, looking at impacts on fertility, survivorship care, and late effects of treatment
- Several BCSC studies, which are focused on identifying factors that drive inequities in breast imaging performance and outcomes, exploring how artificial intelligence can improve outcomes, developing equitable breast cancer risk models, and reducing surveillance imaging failures among women with breast cancer
- Several PROSPR studies, which are looking at ways to improve screening delivery and outcomes for cervical and colorectal cancer
- A cohort study with the University of Wisconsin, which is working to model thyroid cancer incidence and overdiagnosis and understand the role of health care utilization in these outcomes
Past findings include:
- A human-centered-design study in collaboration with Kaiser Permanente Northwest developed a model to help clinical teams talk to cancer patients about cost of care.
- The Systems of Support to Increase Colorectal Cancer Screening and Follow-Up (SOS) study found that mailing cancer screening kits to patients increased regular screening and was more efficient for clinic staff. The research led to implementation of a new program at Kaiser Permanente Washington to help find, treat, and prevent colon cancer.
- Several BCSC studies published new findings on breast cancer overdiagnosis, the benefits and harms of preoperative MRIs, and how best to predict risk of advanced breast cancer.
- Researchers looking at equity in breast cancer screening found that race, income, and education affect access to 3D mammography.
- The ENGAGED-2 study tested a web-based, patient-level intervention that prompted women who had an elevated 5-year breast cancer risk to engage in clinical discussions about chemoprevention.
- The Radiation-Induced Cancers (RIC) study quantified exposure to ionizing radiation in pregnancy over more than 20 years in the U.S. and Ontario, Canada.
- The STOP Colon Cancer in Priority Populations (STOP CRC) program worked with federally subsidized clinic systems caring for low-income and uninsured patients to design a colon cancer screening program that could decrease disparities in screening rates and cancer outcomes.
- A study with the PROSPR consortium found that harms of cancer screening are reported inconsistently across U.S. cancer screening guidelines, and highlighted opportunities to provide better evidence for analysis of harms and benefits to inform screening decisions.
- The PATH trial found that group-based acceptance and commitment therapy could be a viable alternative to standard care for helping people quit smoking.
Recent publications on Cancer
Foster VM, Trentham-Dietz A, Stout NK, Lee CI, Ichikawa LE, Eavey J, Henderson L, Miglioretti DL, Tosteson ANA, Bowles EA, Kerlikowske K, Sprague BL. Supplemental breast cancer screening after negative mammography in U.S. women with dense breasts. J Natl Cancer Inst. 2025 Jun 1;117(6):1271-1275. doi: 10.1093/jnci/djae272. PubMed
Gjesvik J, Moshina N, Lee CI, Miglioretti DL, Hofvind S. Artificial intelligence algorithm for subclinical breast cancer detection. JAMA Netw Open. 2024;7(10):e2437402. doi: 10.1001/jamanetworkopen.2024.37402. PubMed
Di Giuseppe G, Sutradhar R, Pequeno P, Kwan ML, Miglioretti DL, Smith-Bindman R, Pole JD. Medical imaging utilization in migrants compared with nonmigrants in a universal healthcare system: a population-based matched cohort study. PLoS Med. 2024 22;21(10):e1004474. doi: 10.1371/journal.pmed.1004474. eCollection 2024 Oct. PubMed
Figueroa Gray MS, Shapiro L, Dorsey CN, Randall S, Casperson M, Chawla N, Zebrack B, Fujii MM, Hahn EE, Keegan THM, Kirchhoff AC, Kushi LH, Nichols HB, Wernli KJ, Sauder CAM, Chubak J. A patient-centered conceptual model of AYA cancer survivorship care informed by a qualitative interview study. Cancers. 2024 Sep 4;16(17):3073. doi: 10.3390/cancers16173073. PubMed
Miglioretti DL, Abraham L, Sprague BL, Lee CI, Bissell MCS, Ho TH, Bowles EJA, Henderson LM, Hubbard RA, Tosteson ANA, Kerlikowske K. Association between false-positive results and return to screening mammography in the Breast Cancer Surveillance Consortium cohort. Ann Intern Med. 2024 Sep 3. doi: 10.7326/M24-0123. Online ahead of print. PubMed
Researchers in Cancer
 Melissa L. Anderson, MSPrincipal Collaborative Biostatistician |
 Erin J. Bowles, MPHDirector, Collaborative Science |
 Jessica Chubak, PhDSenior Investigator |
 Yates Coley, PhDAssociate Biostatistics Investigator |
 Andrea J. Cook, PhDSenior Biostatistics Investigator |
 Marlaine Figueroa Gray, PhDAssistant Investigator |
 Beverly B. Green, MD, MPHSenior Investigator |
 Laura E. Ichikawa, MSPrincipal Collaborative Biostatistician |
 Ellen O'Meara, PhDPrincipal Collaborative Scientist |
 Lorella Palazzo, PhDSenior Collaborative Scientist |
 Gaia Pocobelli, PhDSenior Collaborative Scientist |
 Rod L. Walker, MSPrincipal Collaborative Biostatistician |
 Robert D. Wellman, MSPrincipal Collaborative Biostatistician |
 Karen Wernli, PhDSenior Investigator |
 Onchee Yu, MSPrincipal Collaborative Biostatistician |
 Weiwei Zhu, MSSenior Collaborative Biostatistician |
 Yu-Ru Su, PhDAssociate Biostatistics Investigator |
 Brian D. Williamson, PhDAssistant Biostatistics Investigator |
 Noorie Hyun, PhDAssociate Biostatistics Investigator |
 Pamela A. Shaw, PhD, MSSenior Biostatistics Investigator |
 Nicole M. Gatto, PhD, MPHPrincipal Collaborative Scientist |
 Meagan C. Brown, PhD, MPHAssistant Investigator |
 Nora Henrikson, PhD, MPHAssociate Investigator |
Affiliate researchers
Wylie Burke, MD, PhD
University of Washington (UW) Department of Medical History and Ethics
Joann G. Elmore, MD, MPH
Harborview Medical Center; UW Department of Epidemiology
Larry Kessler, ScD
UW Department of Health Services
Constance D. Lehman, MD, PhD
Seattle Cancer Care Alliance; UW Department of Radiology
Kathy Leppig, MD
Kaiser Foundation Health Plan of Washington; UW Department of Pathology
Peggy L. Porter, MD
Fred Hutchinson Cancer Research Center (FHCRC); UW Department of Pathology
Emily White, PhD
FHCRC; UW Department of Epidemiology
Rachel Winer, PhD, MPH
Fred Hutchinson/UW Cancer Consortium; UW Department of Epidemiology